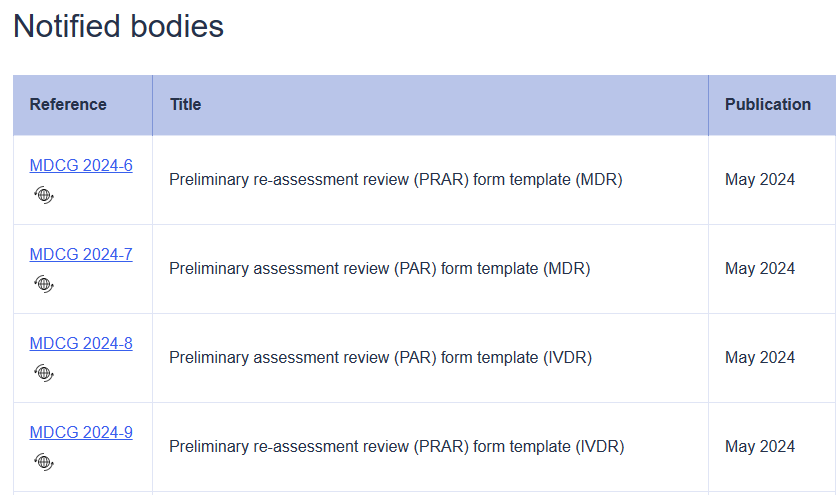
Introduction and scope This document aims to provide guidance to the authorities responsible for notified bodies (hereafter, the Designating Authorities) and joint assessment teams (JATs) when conducting: − assessments of conformity assessments bodies (CABs) that apply for designation as a notified body (NB) in the field of medical devices and/or in vitro diagnostic medical devices,− extensions ..