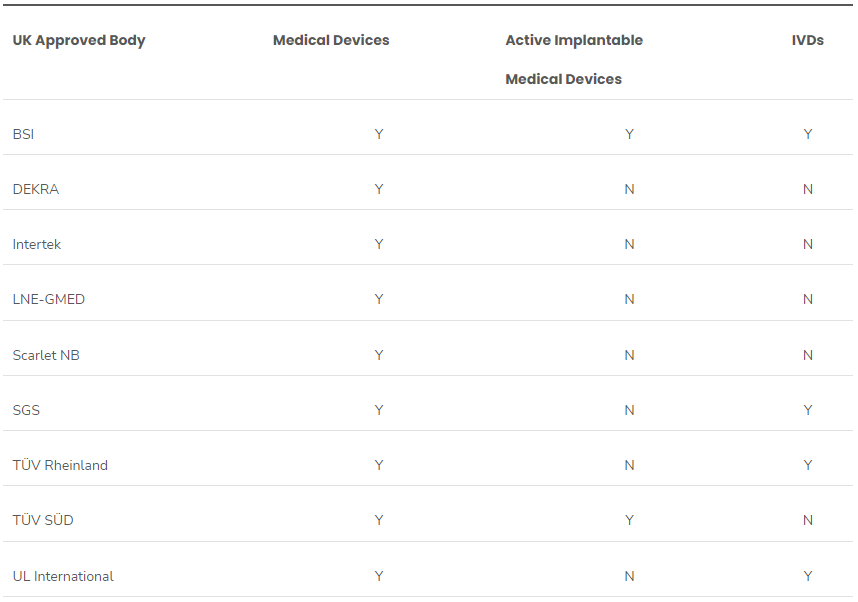
March in Europe The European Commission (EC) updated several medical device safety regulation documents in March 2024. This includes additional standards harmonized with the Medical Devices Regulation (2017/745, MDR) and In Vitro Diagnostics Devices Regulation (2017/746, IVDR) and published in the Official Journal of European Union (OJEU). It also includes an updated study on Notified Body appli..