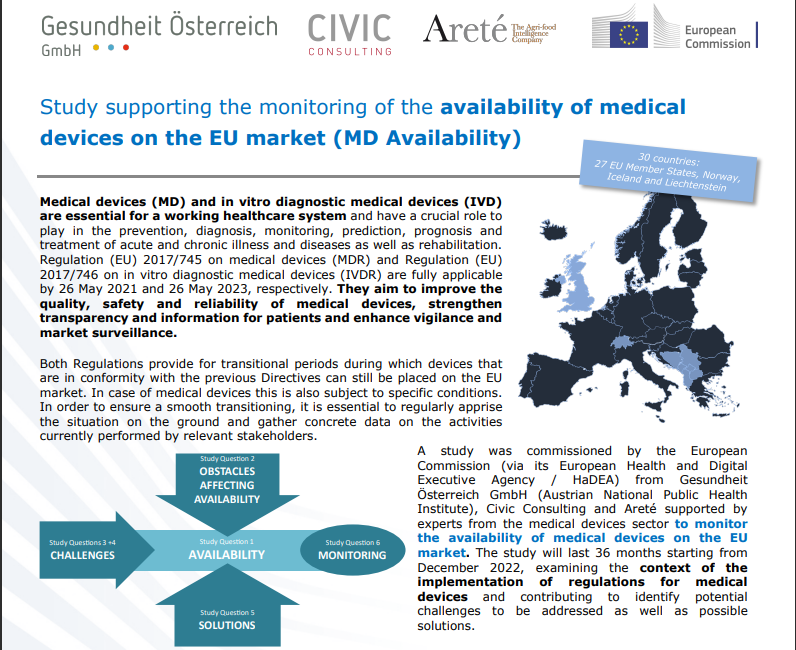
The European Commission’s Directorate-General for Health and Food Safety (DG SANTE) - through the European Health and Digital Executive Agency (HaDEA) - has commissioned a “Study supporting the monitoring of availability of medical devices on the EU market”. The study started in December 2022 and will be running for 36 months (December 2025). The study has been contracted to a consortium led by ..