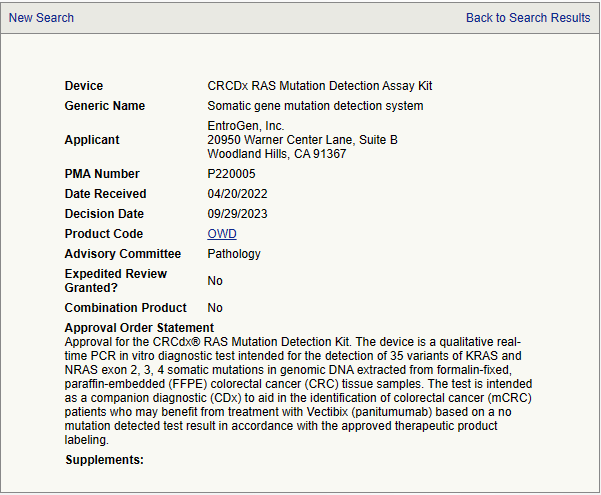
FDA updated of the list of cleared or approved companion diagnostic devices (in vitro and imaging tools). - Device: CRCDx RAS mutation detection assay kit - Generic name: somatic gene mutation detection system - Applicant: EntroGen, Inc - PMA number: P220005 - Approval order statement Approval for the CRCdx® RAS Mutation Detection Kit. The device is a qualitative real-time PCR in vitro diagnosti..