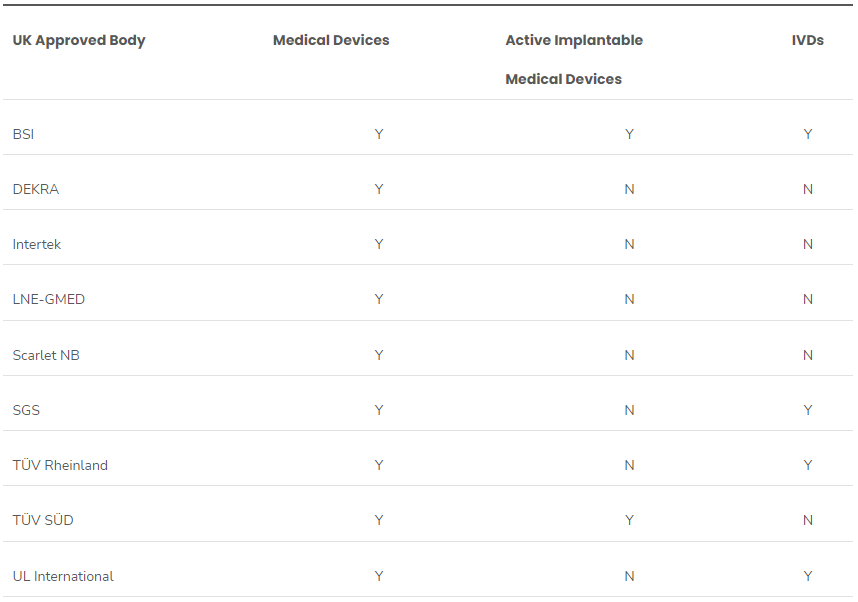
From August 29, 2024, to October 24, 2024, the Medicines & Healthcare products Regulatory Agency (MHRA) held a public consultation on its proposed amendments to the statutory fees. The consultation reflected fees for services related to medicines, medical devices and blood components for transfusion in the UK. The statutory fees for services are intended to recover the costs involved with the wo..