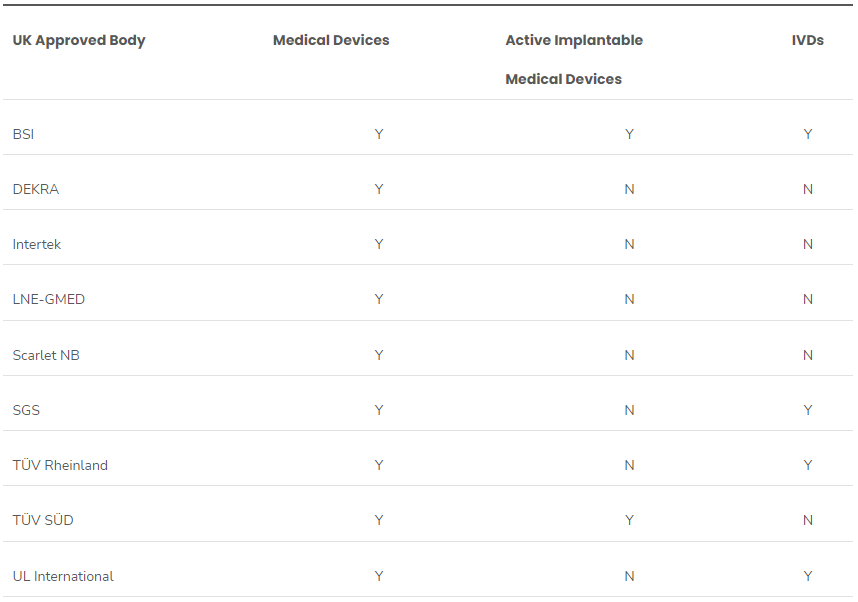
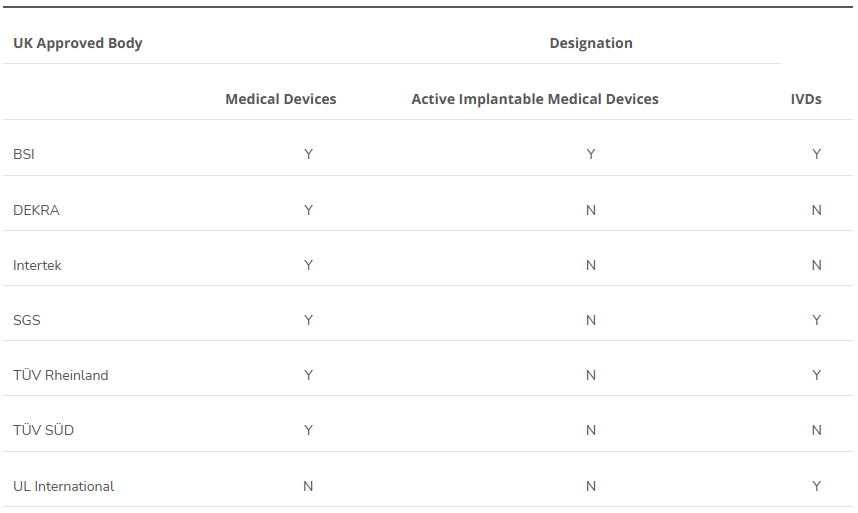
This is the first in our series on the UK MHRA initial draft recognition policy to leverage international regulatory authorizations for access to the medical device market. The UK medical device regulator, the Medicines and Healthcare products Regulatory Agency (MHRA) hosted a webinar in March on the update of the draft medical devices scope, classification and essential requirements. This was a..