FDA에서 최초로 암 유형의 소인을 평가하기 위한 DNA 테스트에 대한 시판 승인을 하였다.
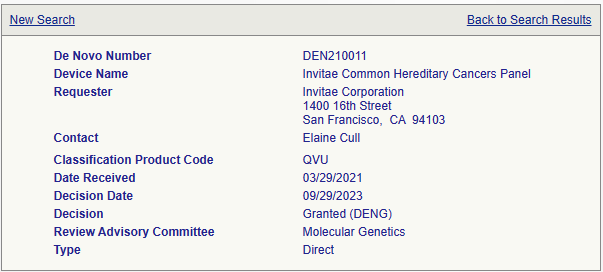
- 업체: Invitae Corporation (미국)
- 제품명: Invitae Common Hereditary Cancer Panel
- 허가: FDA De Novo (DEN210011)
- 제품성능: 9,000개의 임상샘플과 99% 이상의 정확도
- 검체: 혈액
- 47 genes
- 검사기간: 10~21일 (보통 14일)
FDA Grants First Marketing Authorization for a DNA Test to Assess Predisposition for Dozens of Cancer Types
FDA grants marketing authorization for a test that can help detect hundreds of genetic variants associated with an elevated risk of developing certain cancers.
www.fda.gov
Invitae Common Hereditary Cancers Panel | Test catalog | Invitae
Invitae Common Hereditary Cancers Panel | Test catalog | Invitae
Clinical description The Invitae Common Hereditary Cancers panel analyzes 47 genes associated with hereditary breast, ovarian, uterine, prostate, colorectal, gastric, melanoma, and pancreatic cancers. Individuals with a pathogenic variant in one of these g
www.invitae.com


'뉴스 보고서' 카테고리의 다른 글
| [국가생명공학정책연구센터] 미국 '캔서 문샷 (Cancer Moonshot)' 신규 조치 발표 (0) | 2023.10.22 |
|---|---|
| KMDIA 의료기기 배상책임공제 전담부서 '공제사업부' 신설 (0) | 2023.10.20 |
| [KOTRA] 스위스 의료 산업의 미래, 디지털 헬스 (0) | 2023.10.08 |
| [국가생명공학정책연구센터] 개인 맞춤형 의료 제공을 위한 뇌 바이오-디지털 트윈 기술 개발 계획 (0) | 2023.09.28 |
| [NIPA] 주간동향리포트 - 의료부분 AI 도입의 기대와 우려 (0) | 2023.08.25 |