FDA updated of the list of cleared or approved companion diagnostic devices (in vitro and imaging tools).
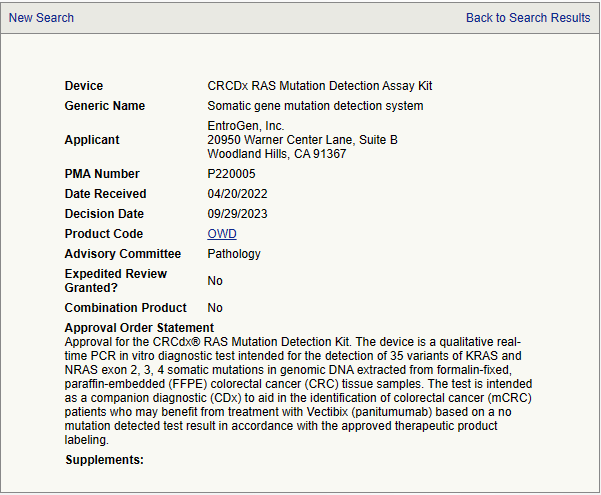
- Device: CRCDx RAS mutation detection assay kit
- Generic name: somatic gene mutation detection system
- Applicant: EntroGen, Inc
- PMA number: P220005
- Approval order statement
Approval for the CRCdx® RAS Mutation Detection Kit. The device is a qualitative real-time PCR in vitro diagnostic test intended for the detection of 35 variants of KRAS and NRAS exon 2, 3, 4 somatic mutations in genomic DNA extracted from formalin-fixed, paraffin-embedded (FFPE) colorectal cancer (CRC) tissue samples. The test is intended as a companion diagnostic (CDx) to aid in the identification of colorectal cancer (mCRC) patients who may benefit from treatment with Vectibix (panitumumab) based on a no mutation detected test result in accordance with the approved therapeutic product labeling.
List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools) | FDA
List of Cleared or Approved Companion Diagnostic Devices
A companion diagnostic device provides information that is essential for the safe and effective use of a corresponding therapeutic product.
www.fda.gov