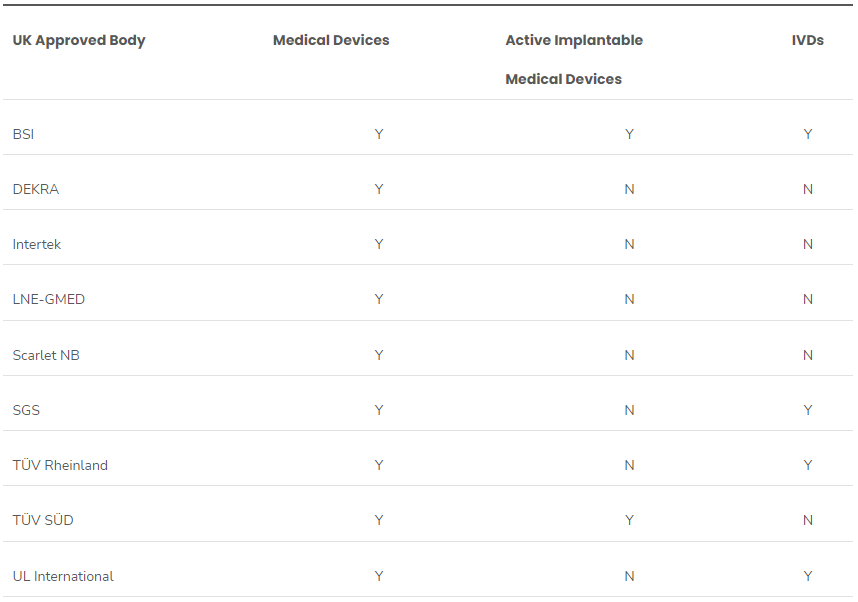
The start of 2024 has seen a flurry of activity from the UK’s Medicines and Healthcare Regulatory Agency (MHRA) regarding medical device legislation.These updates have greatly increased the UK’s capacity to certify medical devices. Since the publication of the UK regulatory roadmap (January 9), there have been several significant updates to the list of UK-approved bodies for medical devices, inc..