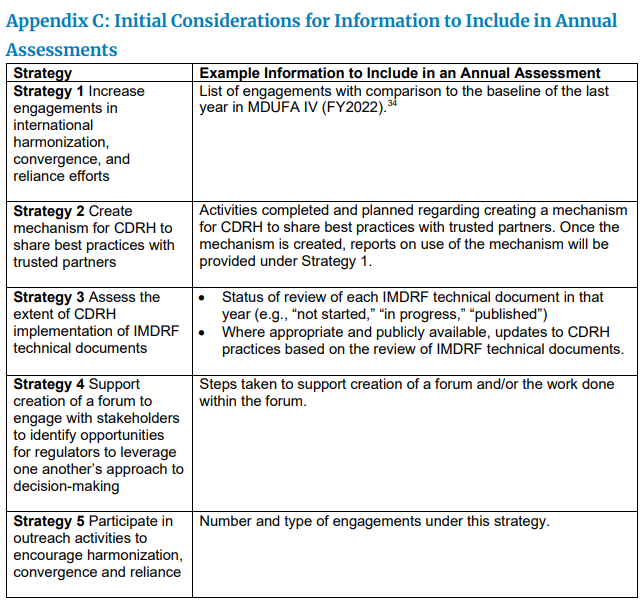
미국 FDA (식품의약품안전처) 내부 CDRH 부서에서 국제규격과 미국 내부 규정과의 조화 (harmonization)를 위한 계획안을 발표하였다 (draft strategic plan).
▶ Mission
It is CDRH's mission to protect and promote the US public health. From an international perspective, this means integrating the US into the international medical device ecosystem. To do so, we develop and foster relationships with other regulatory authorities and strategic partners so that we maintain a current understanding of the evolving global regulatory environment and our role within it. CDRH collaborates with the internaional community to develop and implement harmonized best practices and inform one another's approach to decision-making. We connect internal decision-makers with timely and relevant information to ensure US decisions are made within the context of a global world.
▶ Vision
The US is fully integrated into the international medical device ecosystem so that patients have timely access to high quality, safe and effective medical devices.
CDRH Announces International Harmonization Strategic Plan | FDA
CDRH Announces International Harmonization Strategic Plan
CDRH Announces International Harmonization Strategic Plan
www.fda.gov


'미국 FDA' 카테고리의 다른 글
| [FDA] Cybersecurity in medical devcies Frequently asked Questions (FAQs) (0) | 2023.10.04 |
|---|---|
| [FDA] Cybersecurity in medical devices: Quality system considerations and content of premarket submissions (0) | 2023.10.04 |
| [FDA] Digital Health Frequently Asked Questions (0) | 2023.09.18 |
| [FDA] ISO 10993-1 가이던스 발행 (0) | 2023.09.12 |
| [FDA] 유방암 (변종)에 대한 개인게놈서비스 (PGS) 유전 건강 위험보고서의 마케팅 승인 (1) | 2023.09.04 |