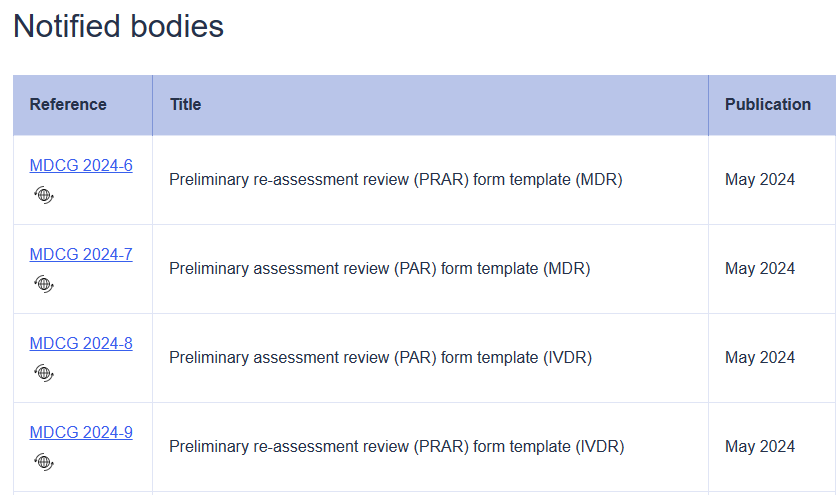
According to Regulation (EU) 2017/746 on in vitro diagnostic medical devices (the IVDR), as part of conformity assessment of class D in vitro diagnostic medical devices (IVDs), the manufacturer must submit an application to a notified body. In addition to the assessment by the notified body, under certain conditions particular elements may be reviewed by an expert panel and/or tested by an EU re..