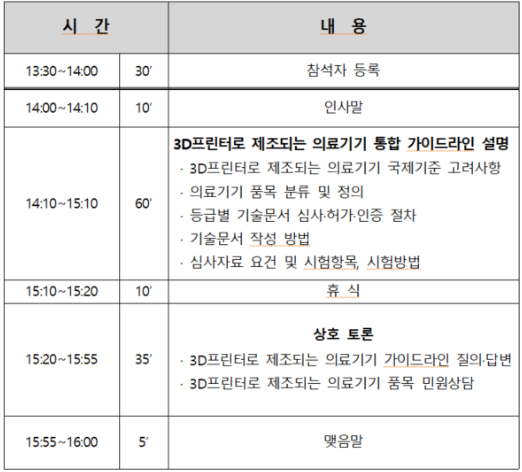
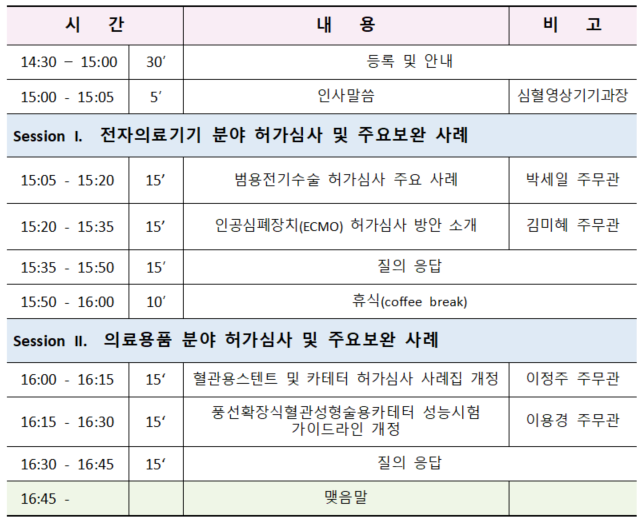
식품의약품안전평가원(재활정형기기과) 에서는 정형용 의료기기 기술문서 심사 시 자주 발생하는 보완 사례를 업계에 공유함으로써 심사의 효율성을 제고하고자 다음과 같이 2024년도 민원설명회를 개최한다. ▶ 일시: 2024년 12월 04일(수) 14:00 ~ 16:00▶ 장소: LW컨벤션센터 중회의실 (서울시 중구)▶ 대상: 정형재활용 의료기기 제조, 수입업체▶ 주제: 정형용 의료기기 주요 품목 보완사례 설명▶ 사전신청: 링크 (11월 27일까지) [식품의약품안전평가원] 2024년도 정형용 의료기기 민원설명회 안내 – 한국의료기기협동조합 [식품의약품안전평가원] 2024년도 정형용 의료기기 민원설명회 안내식품의약품안전평가원(재활정형기기과) 에서는 정형용 의료기기 기술문서 심사 시 자주 발생하는 보와 사례를 업..